I guess we would've seen some reports finding this would be the case, then?
I haven't found any studies of this coming outta any ozone generator.
Giving the hypothesis a shot:
You mean like this: 2 NO + O2 → 2 NO2?
But then this equilibrium follows : 2 NO2 ⇌ N2O4
OK - this will perhaps not "just as easily recombine" as NO2, as one might assume?
For producing NO2 (rather than "just" N2O4, dinitrogen tetroxide), the "elevated temperatures" seems to be crucial.
At room temp the equilibrium above apparently favors N2O4 in what's (eventually) being produced.
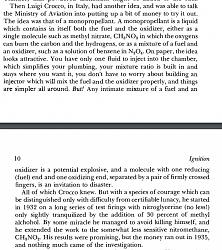
Positive oxidizer, used (with gasoline) by Crocco* in 1930 for liquid rockets,
used extensivly in the US and USSR from the late fifties.
Hypergolic with Aerozine 50, UDMH & MMH, to mention a few:
https://en.wikipedia.org/wiki/Hyperg...pellant#Common
Out of fashion today, replaced by MON because of "reasons":
OTOH: Dinitrogen tetroxide (NTO) isn't something brought from the health store, either:
https://en.wikipedia.org/wiki/Dinitr...o-Soyuz_mishap
I abstain from derailing this thread further, as it pretty quickly would become rocket science,
but for delving further into THAT area I (again) recommend the outstanding biography of John D. Clark: "Ignition!"
(foreword by Isaac Asimov - no less.):
http://www.sciencemadness.org/librar...s/ignition.pdf
*Quote from the book on this Italian intrepid researcher (coincidentally a contemporary compatriot of the Futurists):
ATB
Johan


 LinkBack URL
LinkBack URL About LinkBacks
About LinkBacks


 Reply With Quote
Reply With Quote





Bookmarks