I suspect that like me other machinists keep some caustic soda for things like removing stuck aluminium from carbide milling cutters etc. it really works well.
I had some stored in a plastic jar that started life as a container for honey, jam, coffee or something in that line. A month or so back I wanted to use it, the first time in months. This is what I found.

Click on thumbnails for full size images.
We tend to think of plastic as capable of holding most liquids save for organic solvents, I did but I was wrong.
Glass can be dangerous in a workshop but I keep my caustic soda in it now.


 LinkBack URL
LinkBack URL About LinkBacks
About LinkBacks

 Reply With Quote
Reply With Quote

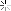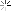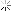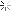


Bookmarks